Knowledge Library
Improving the anti-tumor efficacy of CAR-T through biomolecular condensation
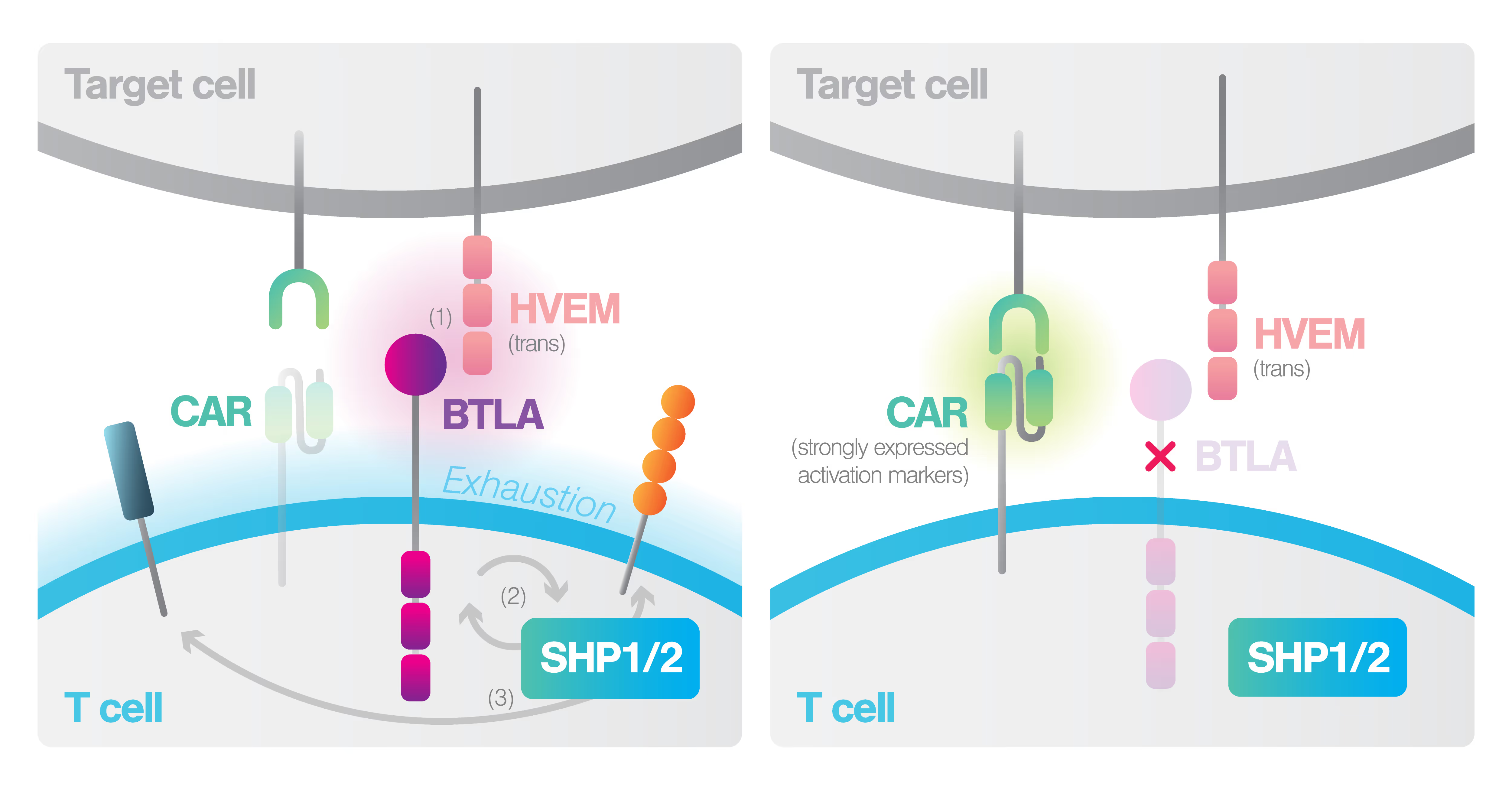
Chimeric antigen receptor (CAR)-T cell therapies showed remarkable efficacies in treating otherwise intractable cancers. However, current clinically approved CAR-T therapies are limited by low antigen sensitivity, impeding their efficacy against cancers with low antigen expression. Here, to address this issue, we engineered CARs targeting CD19, CD22 and HER2 by including intrinsically disordered regions (IDRs) that promote signaling condensation. We discovered that the CAR fused with an IDR from FUS, EWS or TAF15 promoted the formation of CAR-T conjugation with cancer targets, the mechanical strength of CAR-T synapses and membrane-proximal signaling, which led to an increased release of cytotoxic factors and a higher killing activity toward low-antigen-expressing cancer cells in vitro. Moreover, the IDR CAR-T displayed improved antitumor effects in both blood cancer and solid tumor models. No elevated tonic signaling was observed. Together, our work demonstrates IDRs as a new toolset for improving CAR-T function through inducing biomolecular condensation.
This webinar is in Chinese. 此次线上研讨会为中文讲座
Cell Avidity Uncovers Novel KIR:HLA binding predicting patient survival post stem cell transplant
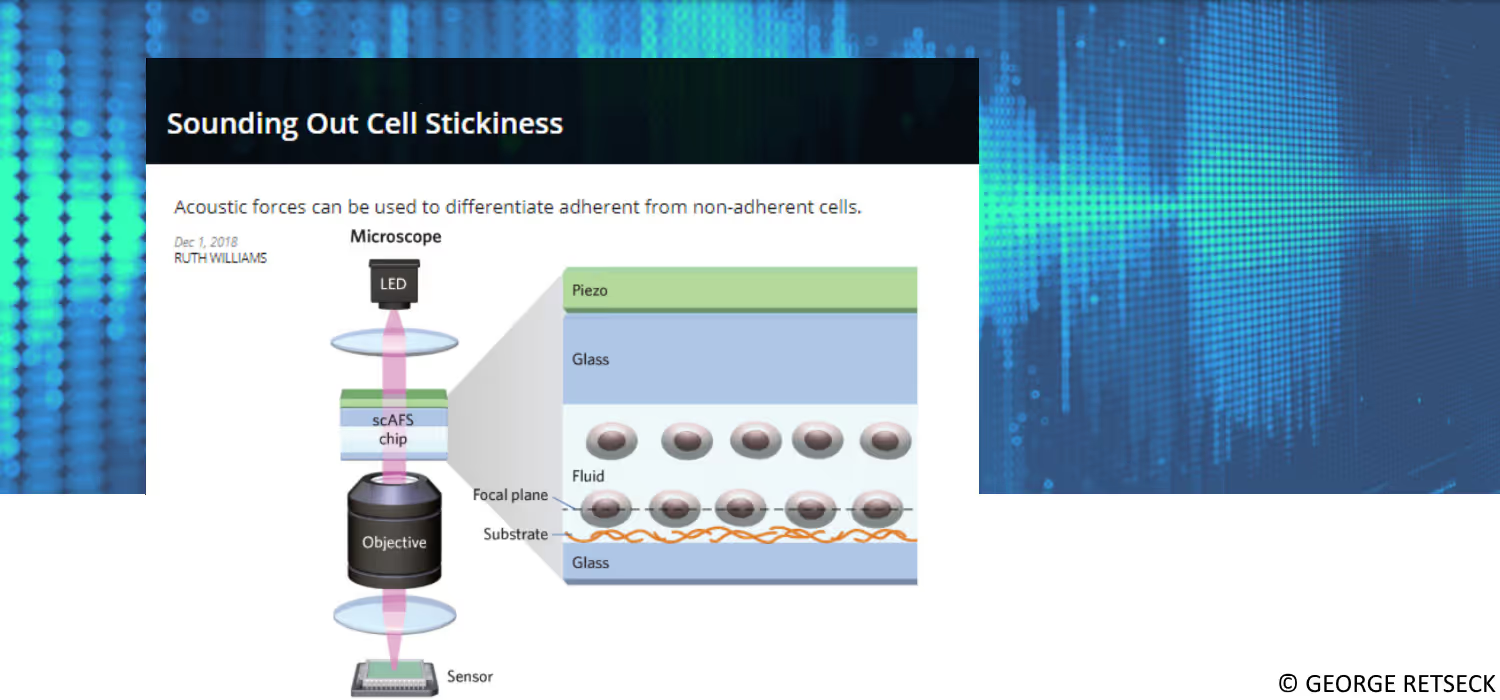
Despite significant progress in haploidentical hematopoietic cell transplantation (haploHCT) for leukemia, relapse-free survival remains a major challenge, even with the addition of immune effectors such as NK or T cells. Given the crucial role of NK cells and their killer immunoglobulin receptors (KIRs) in early anti-leukemic activity, this study sought to refine the understanding of KIR:HLA (human leukocyte antigen) interactions in order to better predict patient outcomes.Using a combination of in silico protein folding and interaction modeling with in vitro acoustic force microscopy to quantify immune synapse avidity, Peter's group identified and validated a novel functional interaction between full-length KIR2DS4 and HLA-B*35. This interaction was confirmed through cell avidity assays with monoallelic KIR and HLA cell lines. When applied to clinical data, patients who received haploHCT and NK cell addback from donors expressing only full-length KIR2DS4 showed significantly improved overall and relapse-free survival. These findings were independently validated in an adult cohort, revealing consistent survival advantages.This newly defined KIR2DS4:HLA-B*35 axis represents an immediately actionable marker for donor selection and offers a mechanistic insight into optimizing NK cell-mediated anti-leukemic responses.
Rational CAR design: Integrating affinity and tonic signaling
The success of chimeric antigen receptor (CAR) T cell therapy for hematological malignancies has not yet translated into long-term elimination of solid tumors, indicating the need for adequate tuning of CAR T cell functionality. The CAR binding moiety is the critical trigger for CAR T cell signaling. CAR binding affinity alone does not determine T cell effector functions. In a panel of anti-Her2 CARs covering a 4-log affinity range, we observed that rather high affinity and cell avidity above the minimum threshold, combined with elevated tonic signaling, produce adequate T cell capacity for expansion and tumor control. The same scFv mutations increased both antigen-specific affinity, cell avidity, and antigen-independent tonic signaling; above a minimum threshold, raise in affinity translated into cell avidity in a non-linear fashion. In this case, replacement by amino acids of higher hydrophobicity within the scFv coincidentally augmented affinity, non-specific binding, spontaneous CAR clustering, and tonic signaling, all together relating to T cell functionality in an integrated fashion. Data highlight the mechanistic complexity of CAR signaling and suggest inclusion of additional variables, for example, hydrophobic interactions, into the equation when determining the CAR’s antigen-specific and tonic signaling capacities.
Identification of potent biparatopic antibodies targeting FGF receptors in solid tumours
Translocations involving FGFR2 gene fusions are common in cholangiocarcinoma and gastric carcinoma and predict response to FGFR kinase inhibitors. However, response rates and durability are limited due to the emergence of resistance, typically involving FGFR2 kinase domain mutations, and to sub-optimal dosing, relating to adverse drug effects.
This webcast will present new work showing that the vast majority of such alterations retain the extracellular domain (ECD), potentially enabling highly selective targeting of the FGFR2 ECD using biotherapeutics.
To improve on the activity of traditional bivalent monotopic antibodies, the Sellers lab systematically generated biparatopic antibodies targeting distinct epitope pairs in FGFR2 ECD, and identified antibodies that effectively block signaling and malignant growth driven by FGFR2-fusions.
These antibodies robustly blocked proliferation and colony formation in FGFR2-fusion driven cholangiocarcinoma and demonstrated robust in vivo anti-tumour activity. In vivo activity was marked by significant antibody-mediated downregulation of FGFR2 and in turn this was associated with robust lysosomal internalization enacted by the two biparatopics. In vitro, the biparatopic antibodies demonstrated activity against FGFR inhibitor resistant alleles of FGFR2. The internalization properties of the antibodies also make them suitable for exploration as antibody-drug conjugates
Cell Avidity measurements to understand and enhance CAR-T/tumor interactions in acute myeloid leukemia
CAR-T therapy can be a highly effective treatment for certain blood cancers such as acute myeloid leukemia (AML), but the mechanisms underlying productive CAR-T cell/tumor interactions are only beginning to be understood. Recent work has demonstrated that productive interactions can be influenced by the density of the target antigen on the tumor cell, stability of the CAR molecule itself, and additional antigen-independent and antigen-regulated interactions mediated by adhesion molecules. The sum total of the T-cell interaction with an antigen-bearing target cell is known as avidity. Watch this webinar from Marcela Maus to learn about novel CARs and the use of avidity measurements to understand and enhance productive CAR-T cell interactions with both liquid and solid tumors.
Cell Avidity as a crucial biomarker for CAR T response
Liquid and solid tumors differ in their interactions with CAR T cells and it is important to understand the critical factors associated with tumor escape mechanisms. In this webinar, Dr. Rebecca Larson presented her work on “Loss of IFNγR signaling and downstream adhesion confers resistance to CAR T cell cytotoxicity in solid but not liquid tumors” in Nature, and suggests that enhancing binding interactions between T cells may yield improved responses in solid tumors. Learn how cell avidity measurements can improve CAR T cell response in solid tumors by identifying important evasion mechanism.
Enhanced avidity through combining a BCMA CAR and a CD38 chimeric co-stimulatory receptor leads to enhanced T cell sensitivity and persistence
B-cell maturation antigen (BCMA) is a potential target antigen in multiple myeloma due to its highly selective expression in malignant plasma cells. BCMA-targeted CAR T-cell therapy has been proven to be effective in patients with multiple myeloma. Despite that complete remission level reaching around 60-86%, a subset of patients still experience tumor relapse. Tumor escape due to low antigen density and limited efficiency in single-targeting BCMA CAR. In this webinar, Dr. Maria Themeli showed how to overcome low antigen density and improve CAR-T cell persistence with multi-targeting and co-stimulation through increasing synaptic avidity with the z-Movi.
Exploring the network of cellular interactions of Natural Killer cells in the tumor microenvironment with cell avidity
NK cells serve as a potential immunotherapeutic tool due to their innate ability to kill cancer cells. They play a crucial role in cancer prevention by providing immune surveillance, making them attractive candidates for immunotherapeutic tool. However, quite often NK cell can fail to recognize and eradicate residual cancer cells, resulting in cancer relapse and lower chance of survival. In this webinar, Prof. Mark Lowdell, Chief Scientific Officer of INmune Bio, Inc. discussed how primed NK cells become effective against resistant tumor cells, and how cell avidity measurements were used to depict a stronger binding of primed NK cells, resulting in improved tumor cell killing.
Dual-targeted cell therapy targeting BCMA and GPRCD5 to prevent relapse in multiple myeloma
Multiple myeloma is a cancer derived from malignant plasma cells. Conventional mono-targeting CAR T-cell therapies targeting BCMA have yielded remission in treated patients, however, a large proportion relapses due to BCMA antigen escape as a result of low antigen density. The development for effective CAR T-cell therapy with long-term remission is needed.
Learn from Prof. de Larrea how expressing two CARs on a single cell enhances the strength of CAR T-cell/target cell interactions, prevents BCMA escape-driven relapse, and how cell avidity measurements contributed to their findings in this webinar.
Unveiling a one-to-one relationship of TCR-dependent T cell function and cellular avidity at single-cell level using acoustic force
TCR T cell therapy is a powerful tool that can redirect patients’ immune cells to target cancer cells. Conventional assays such as affinity measured by SPR do not always correlate with in vivo results, so the selected candidates might turn out not to be the best one. Our cell avidity expert, Dr. Will Singleterry, will present the insights cell avidity experiments provided for the team of Dr. Nathalie Rufer at University of Lausanne in this webinar.
TCR- and BsAb-induced interaction drives avidity-based cellular immunotherapy efficacy
Bispecifc antibodies (bsAb) are molecules that can steer immune cells, such as T cells, NK cells, towards cancer cells. They facilitate the formation of the immunological synapses (IS), which are key driver of downstream T cell response. In this webinar, our principal scientist will show how cell avidity measures the effectiveness of the events during IS formation, and allow researchers to rank and select the best candidates.
How Cell Avidity between tumor-effector cell pairs drives the efficacy of cellular immunotherapy
CAR-T cell therapy has increased in its popularity due to its robust nature and being MHC-independent. In this webinar, Dr. Rogier Reijmers, Principal Scientist at LUMICKS, will present several case studies and demonstrate how measuring Cell Avidity provides new insights by providing a more complete and physiologically relevant picture of the interaction between cells and their targets.
Cell avidity of CAR-NK cells revealed as the best and earliest predictive parameter for 𝙞𝙣 𝙫𝙞𝙫𝙤 tumor control
Recent work using cell avidity measurements by the Gottschalk lab at St. Jude Children’s Research Hospital (published in Nature Biotechnology) sought to tune CAR synapses in NK cells by adding an intracellular scaffolding protein binding site to the CAR called the PDZ binding motif.
Improving CAR-T immunotherapy for lymphoma by fine-tuning avidity
Commercial CAR-T therapies still suffer from severe limitations, as majority of patients fail to achieve complete response and ultimately relapse.
Watch this Webinar to discover Prof. Marco Ruella’s team have adopted a novel CAR-T avidity screening method to improve safety and exhaustion profile, leading to 100% clinical response in a phase I trial.
Understanding the mechanism of action of a novel dual CAR approach with improved efficacy and safety using Cell Avidity
Acute myeloid leukemia (AML) continues to be an unmet clinical need for both adult and pediatric patients. Although CAR-T cell therapy has demonstrated substantial therapeutic potential, further advancements are necessary to achieve safe and lasting disease remission. In this webinar, CAR-CIK cell pioneer Dr. Sarah Tettamanti, from the Tettamnti foundation in Milan, Italy (alongside our Lead Product Manager, Shira Segal, at LUMICKS) discuss how Cell avidity was used to generate an additional layer of information to understand the mechanism of action and enhance decision-making on identifying the most efficacious candidates whilst limiting toxicity to healthy cells.
Enhancing efficacy against clear cell renal cell carcinoma through format-tuning of bispecific T cell engagers
Cell Avidity uncovers critical binding mechanism impairing T-cell potency in the Tumor Microenvironment
Cell Avidity screening deployed by T cell pioneers to accelerate the identification of functional TCRs
Cell Avidity-tuning ensures safe CAR-T therapy for solid tumors
From Handshakes to Hugs: Embracing the Dynamics of Immunological Synapse Strength and Avidity
Measure interaction strength between target cell and TCR T-cell in HPV-related cancers with the z-Movi®
Cell avidity is a superior predictor of CAR T-cell efficacy in myeloma than cytotoxicity – first published z-Movi®results
Screening of cancer targeting TCRs with the z-Movi®
Rapid assessment of CAR T-cell strategies for multiple myeloma with Cell Avidity analysis
The Scientist Magazine features z-Movi
z-Movi featured in Science
Unraveling the Kinetics and Strength of T Cell Adhesion to Fibronecting using z-Movi®;
High-Throughput Label-free Cell Interaction Studies
Rapid assessment of CAR T cell strategies for multiple myeloma with the z-Movi Cell Avidity Analyzer
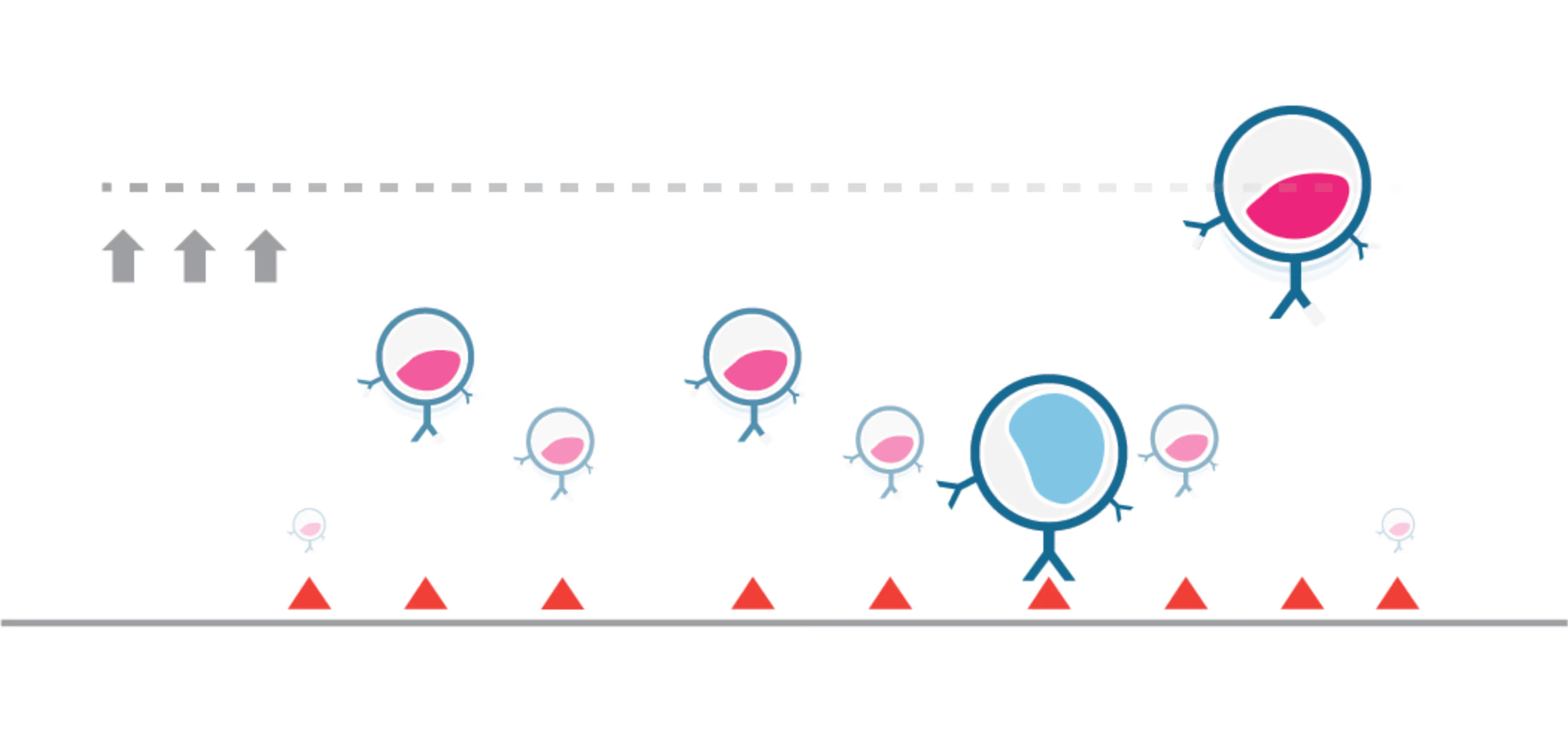
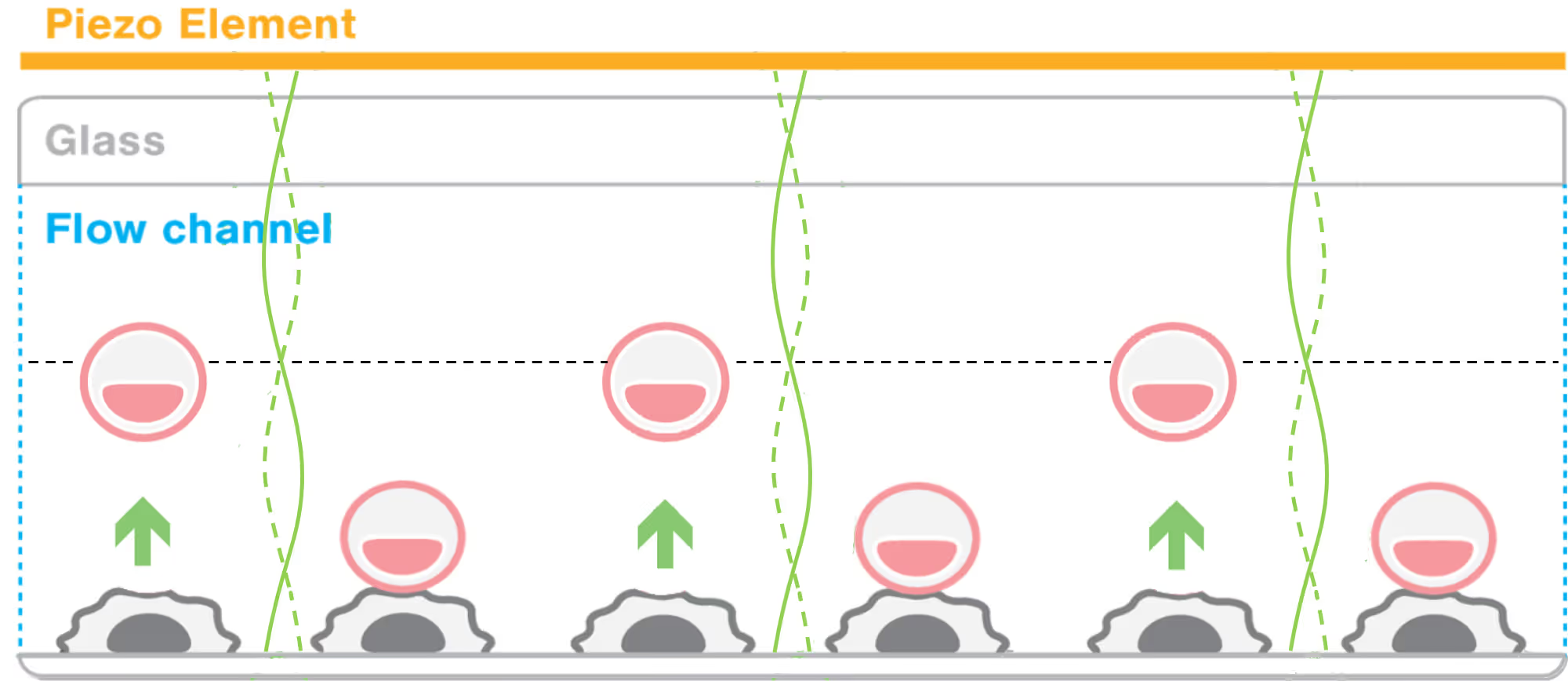
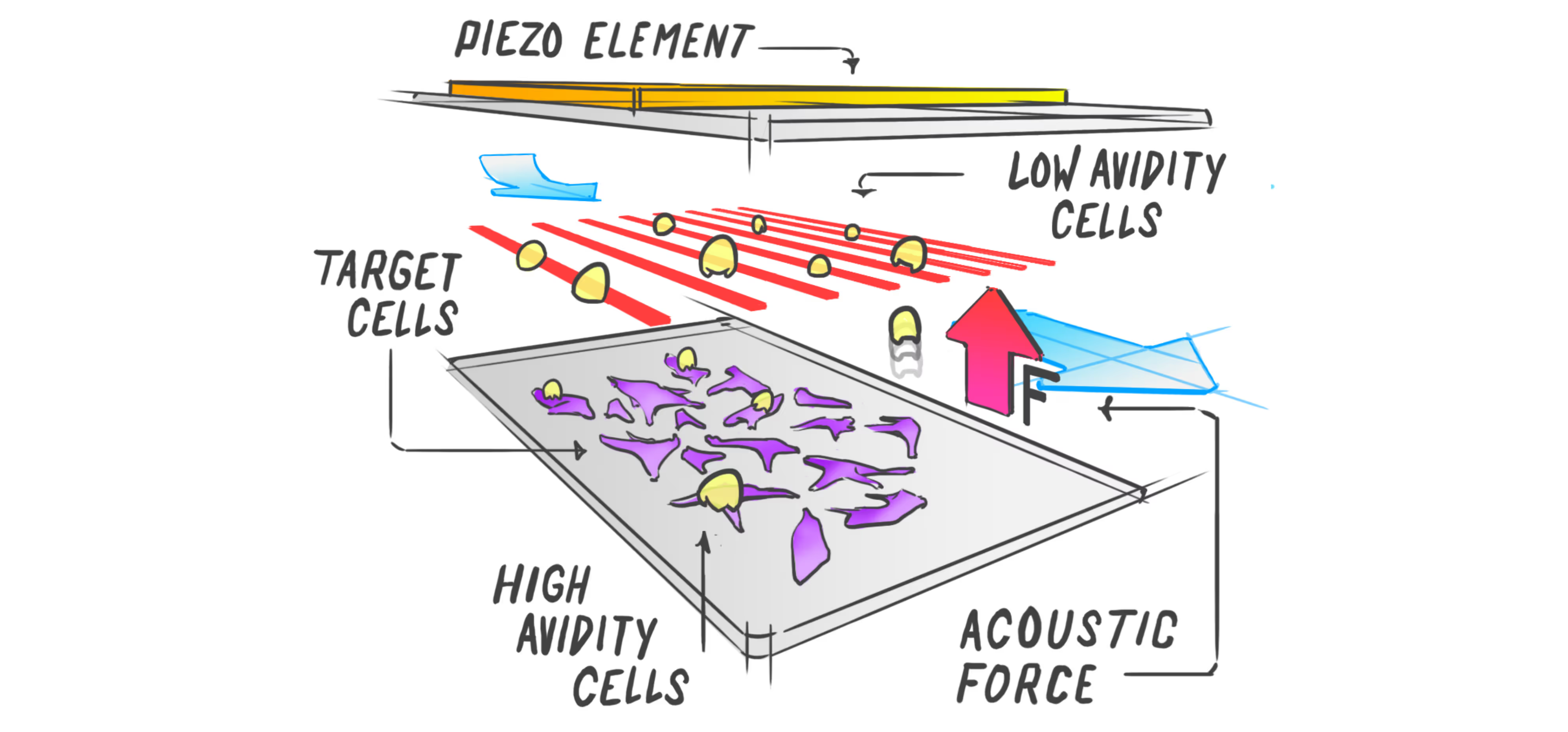
Identifying effective immunotherapeutic treatment strategies for multiple myeloma that also mitigate relapse often requires tedious and time-consuming validations, such as cell-killing assays and in vivo engraftments. We show that intercellular binding strength (cell avidity), measured by the z-Movi® Cell Avidity Analyzer, quickly predicts CAR T-cell efficacies that correlate with treatment outcomes in vivo.
Identify functionally optimal TCR T cells through Cell Avidity measurements
T cell receptor (TCR) -based cancer immunotherapy has the potential to become a powerful approach to treat solid tumors, such as melanoma. However, conventional methods that validate the effectiveness of TCR transduced T cells are often inconsistent with functional assays or are tedious to perform. In this application note, we show how the z-Movi® Cell Avidity Analyzer reliably and quickly identifies functionally optimal TCR-engineered T cells targeting melanoma cell lines based on cell–cell interaction strength (cellular avidity).
Accelerate your cell engager discovery with high throughput measurements of Cell Avidity
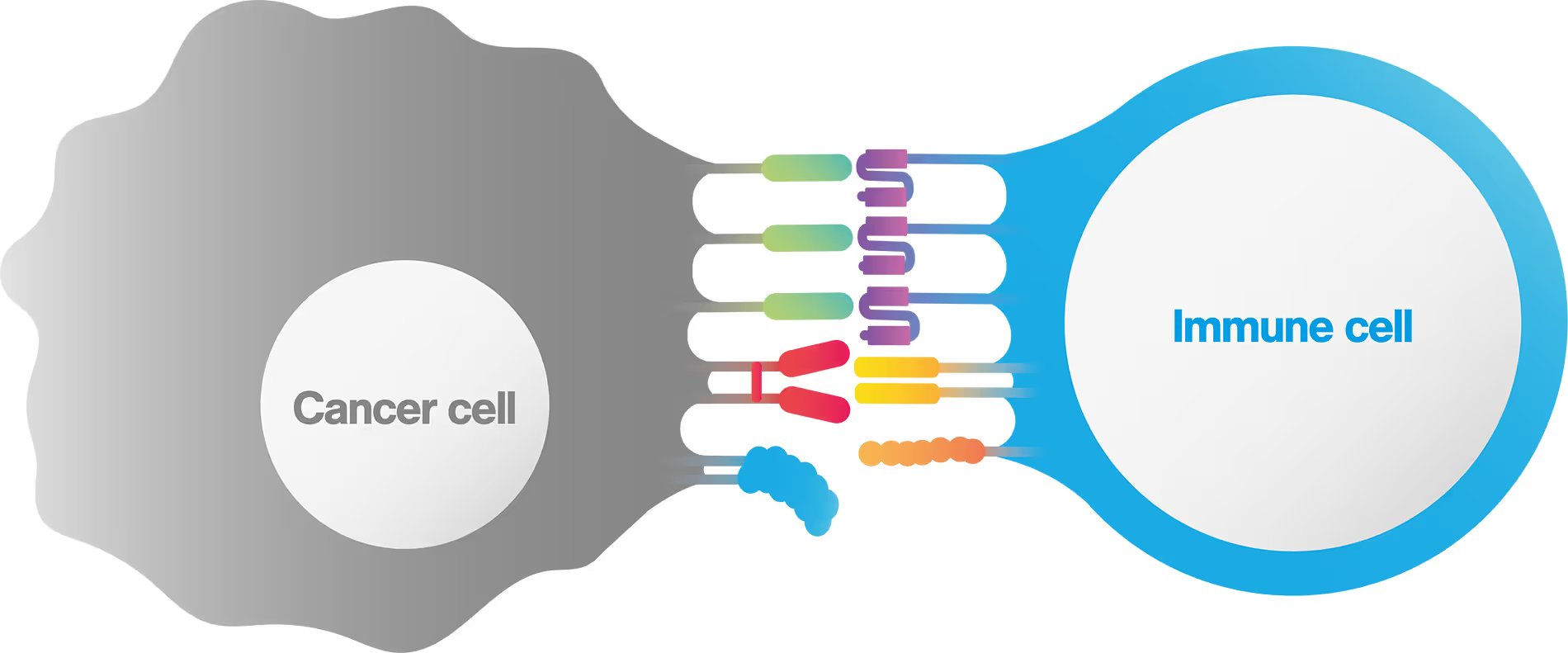
T cells play a pivotal role in tumor immunosurveillance. Multispecific cell engagers (CEs) have been adopted in the field of immuno-oncology to redirect T cells toward cancer cells, thereby unleashing the anti-tumor potential of the patient’s immune system. CE-mediated cell binding induces T cell activation and the formation of an immunological synapse, which is a prerequisite for effective tumor cell lysis.
The strength of the initial binding events between a T cell and a tumor cell dictates the efficiency of the anti-tumor response. Assessing cell avidity, i.e. the total intercellular interaction strength between two cells, gives crucial insights into the efficacy of CEs as anti-tumor therapeutic agents.
Here, we deploy LUMICKS’ high throughput avidity measurement (HTAM) technology to measure CE-induced cell avidity in a high throughput manner. We demonstrate the assay performance characteristics, i.e. specificity, precision, and range, via CE titration experiments in the context of a Jurkat T cell model system. We find that the HTAM CA assay is suitable for candidate screening in high throughput, with high sensitivity and precision.
Accelerate your cell engager discovery with high throughput measuremenets of Cell Avidity
Cell Avidity: a key to accelerate IND filing in cell therapy drug development
Cell Therapy Case Study Collection
z-Movi Product Brochure
Avidigo Service Brochure
Cell Avidity Technology Brochure
Avidion Product Brochure