Variations in low-complexity domains and how they influence protein droplet solidification
Here, researchers from the lab of Dr. Bo Sun at ShanghaiTech assessed how variations in low-complexity domains influence droplet solidification. The investigators used force-induced droplet fusion to evaluate peptide droplet properties. They evaluated the influence of low-complexity domain cores within hnRNPA1 proteins called reversible amyloid core (RAC).
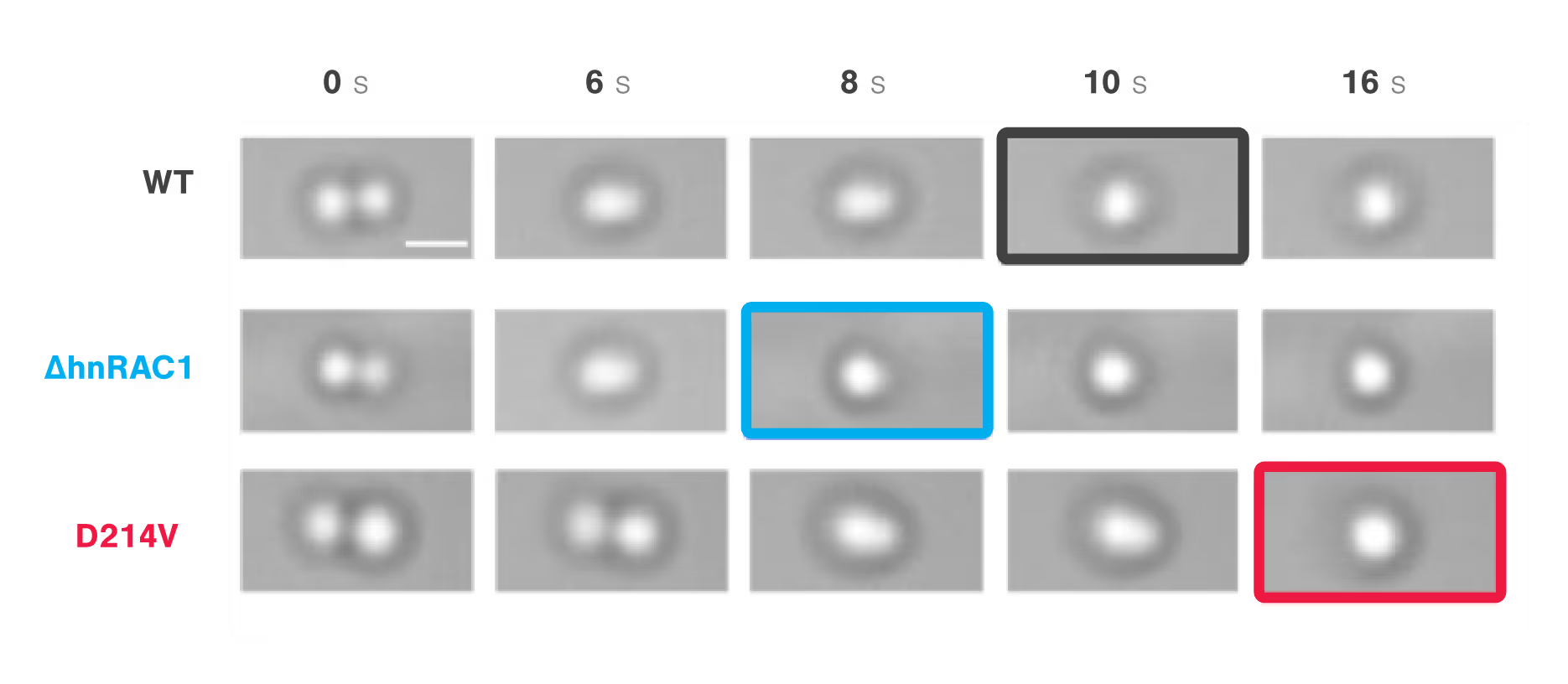
Compared with wild-type hnRNPA1 droplets, the deletion of hnRAC1, a specific segment within the protein, resulted in faster droplet fusion (10 seconds and 8 seconds, respectively). Conversely, hnRAC1 missense mutation of the aspartic acid to valine (D214V; associated with irreversible amyloid formation) resulted in an even slower droplet fusion (16 seconds; Figure 1).
Data courtesy of Dr. Bo Sun at ShanghaiTech
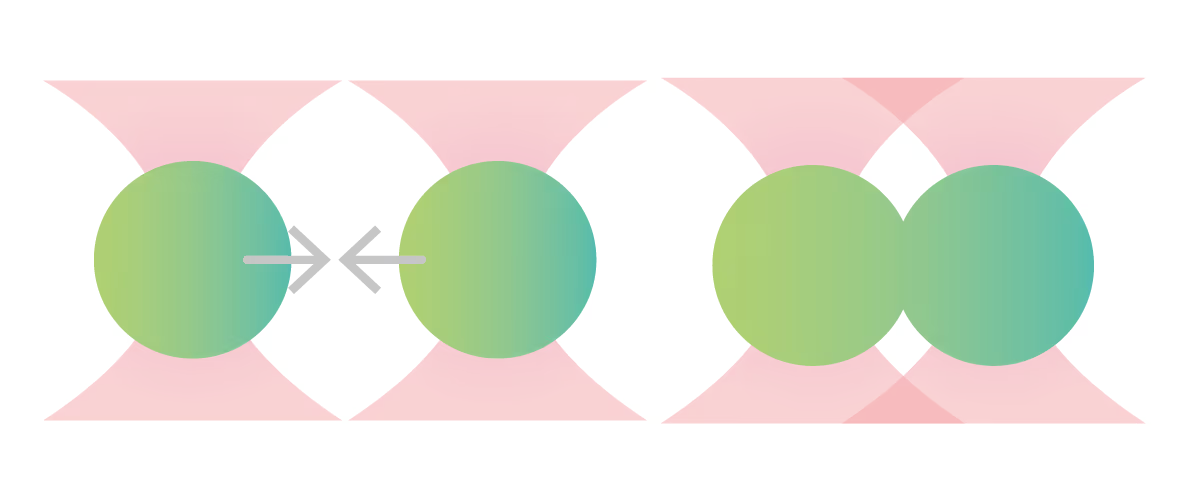
Figure 1: Time-lapse images comparing the times to relaxation of protein droplets consisting of wild type (WT), RAC-depleted (ΔhnRAC1), or RAC-mutated (D214V) during optical tweezer-induced droplet fusion. Highlighted images indicate the time point of droplet fusion. Image source: Gui et al, Nature Communications, 2019 (CC BY).
Dive into the publication
Explore further

Manipulate and study protein droplet dynamic and properties in realtime to understand phase separation
In this application note, we highlight multiple experiments conducted at Banerjee Lab at the University at Buffalo, and Hyman Lab at the Max Planck Institute of Molecular Cell Biology and Genetics on phase separation using the C-Trap dynamic single molecule technology. We present the two approaches the studies used to assess specific properties of protein droplets through applied forces.
The effect of molecular crowders on droplet fusion
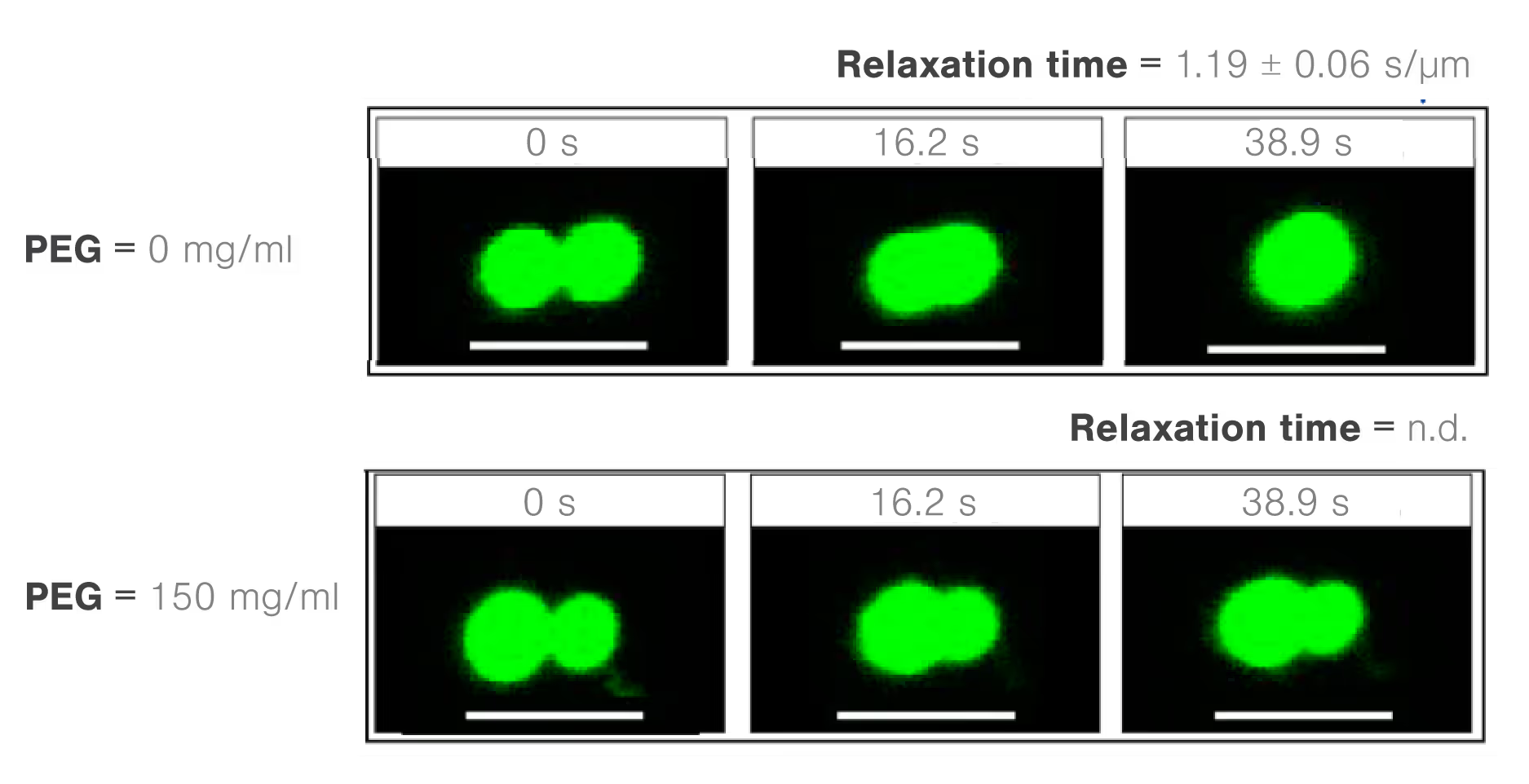
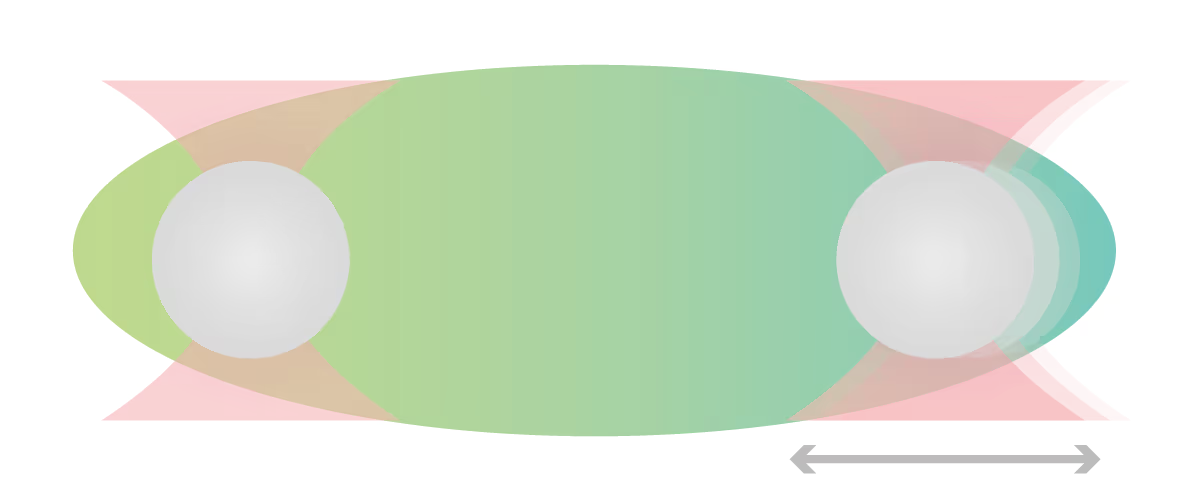
Using the polyethylene glycol PEG8000 as a model structure, researchers from the Banerjee lab measured the concentration-dependent effect of molecular crowders on the properties of FUS protein droplets. They moved one of two trapped FUS droplets towards the other at a constant velocity and timed their fusion (time-to-relaxation) at different PEG8000 concentrations.
While the absence of PEG8000 crowders resulted in a fast droplet fusion (about 200 ms/μm), the presence of 150 mg/ml PEG8000 almost arrested the condensation (Figure 1).
Data courtesy of Dr. Priya Banerjee at the University at Buffalo

Dive into the publication
The effect of RNA/RNP interactions on the fusion of protein droplets
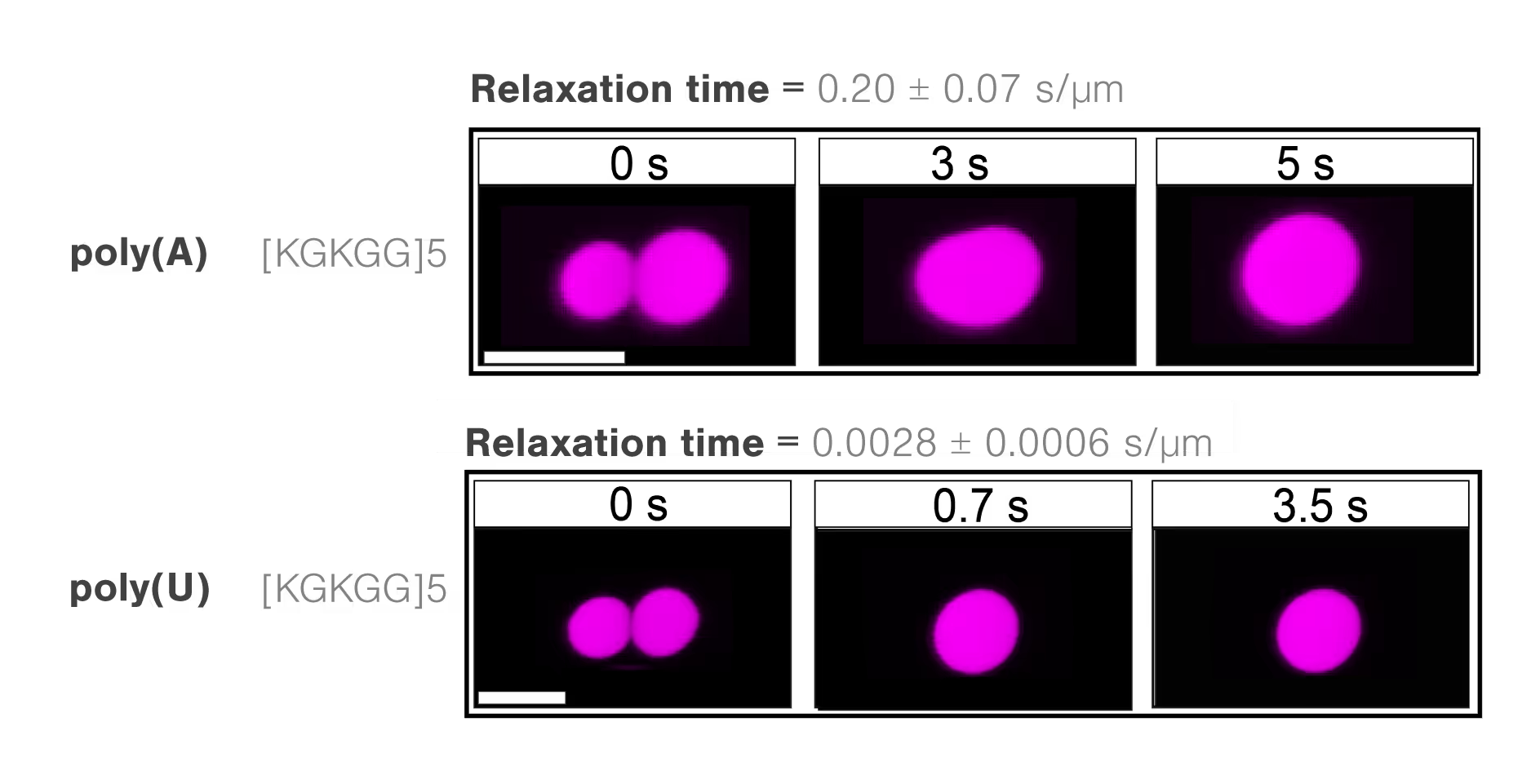
Scientists from the Banerjee lab at the University at Buffalo investigated the condensation properties between different RNAs and polypeptides. The researchers assessed the molecule-dependent effect of interactions between poly(A) or poly(U) RNAs and different peptide domains (R/G-rich or K/G-rich) on droplet stability, measured through droplet fusion.
They found that peptide droplets composed of K/G-rich peptides and poly(U) fused twice as fast (mean, 0.0028 s/µm) as droplets with R/G-rich peptides and poly(A) RNA (Figure 3). The results indicate a higher fluidity and lower viscosity in the former structure and suggest that short-range attractions and long-range forces regulate RNA–peptide condensate formation.
Data courtesy of Dr. Priya Banerjee at the University at Buffalo
Dive into the publication
Microrheology, viscosity, and elasticity of protein droplets upon increasing salt concentrations
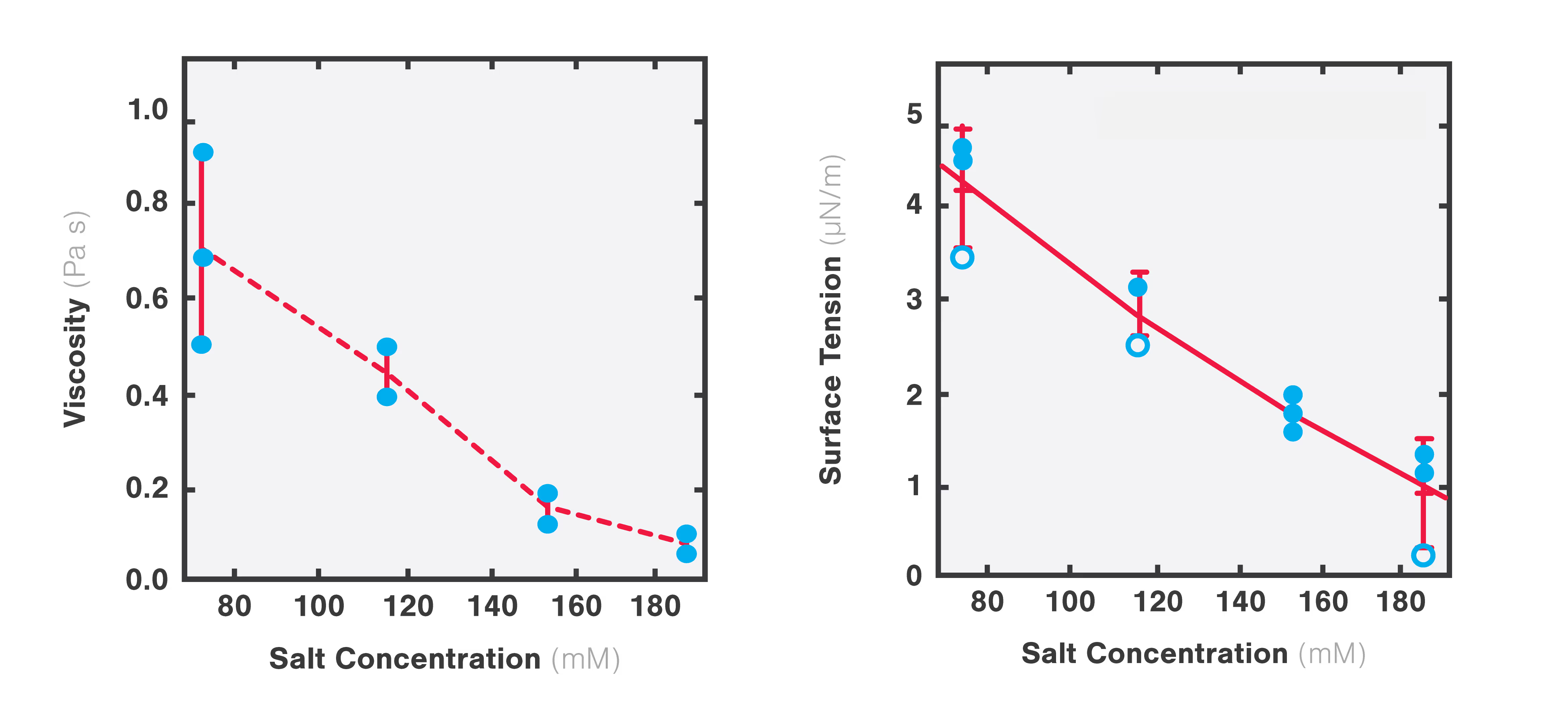
Using two optical traps, scientists from the Hyman lab at the Max Planck Institute of Molecular Cell Biology and Genetics could study the microrheology of protein droplets consisting of PGL-3 proteins in different salt concentrations (75, 115, 150, and 180 mM KCl).
They brought two independently trapped beads into adhesive contact with the droplet and applied force on one bead, moving it bi-directionally to deform the droplet. Since the forces needed to move the trap changes with altered thickness, they could measure the structure’s viscosity as a result of different salt concentrations.
The team found that both the viscosity and surface tension of the protein droplet decreased with increasing salt concentrations (1 to 0.1 Pa s and 5 to 1 µN/m, respectively: Figure 1).
Data courtesy of Prof. Dr. Anthony Hyman at the Max Planck Institute of Molecular Cell Biology and Genetics
Dive into the publication
These cards are NOT components because they use the finsweet nested collection logic. To pull in the posible multiple people wo worked on it.
This type of 1-many relation is not supported native in Webflow.
Also this section is hidden when emtpy. To keep everything visible here, that is being done outside the webflow designer from within Slater.
FUS Oncofusion Protein Condensates Recruit mSWI/SNF Chromatin Remodeler via Heterotypic Interactions Between Prion-like Domains
Single-stranded nucleic acid binding and coacervation by linker histone H1
Homotypic RNA clustering accompanies a liquid-to-solid transition inside the core of multi-component biomolecular condensates
SITC 2025
CAR-TCR Summit 2025
CICON 2025