Keeping up with the complexity of binding mechanisms
- Generate direct, physiologically relevant measurements of binding in its full, dynamic complexity
- Understand the mechanism of action of your therapeutic products by revealing its complex binding dynamics
- Balance potency and safety by optimizing binding
Cell Avidity measures true binding of biparatopic antibodies
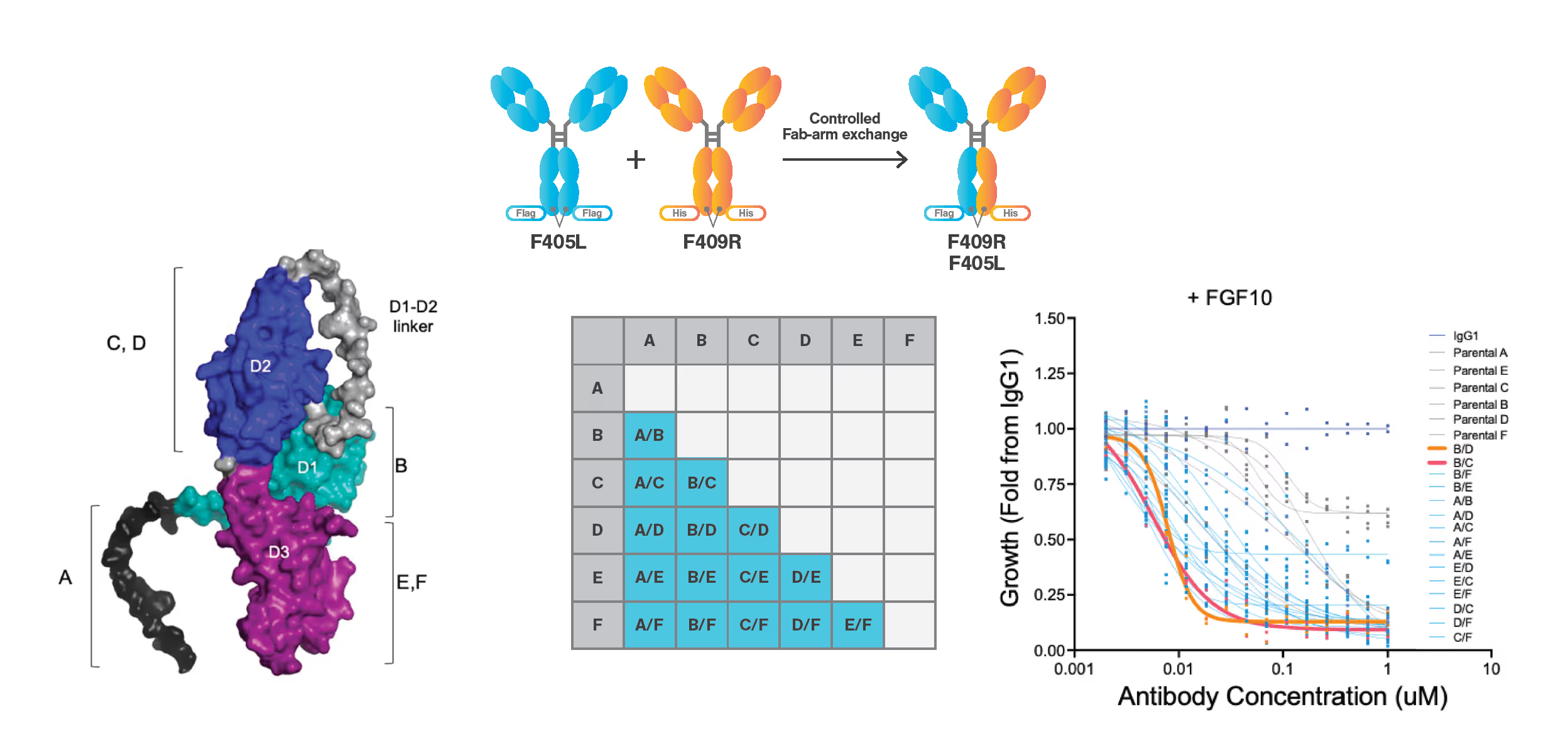
Mechanistic issues limit the effectiveness of many current cancer-targeting antibody therapies, with monospecific antibodies often hindered by receptor dimerization and activation. Biparatopic antibodies, which bind to two unique non-overlapping epitopes, offer a promising solution with stronger binding, more potent antagonism, and higher specificity.
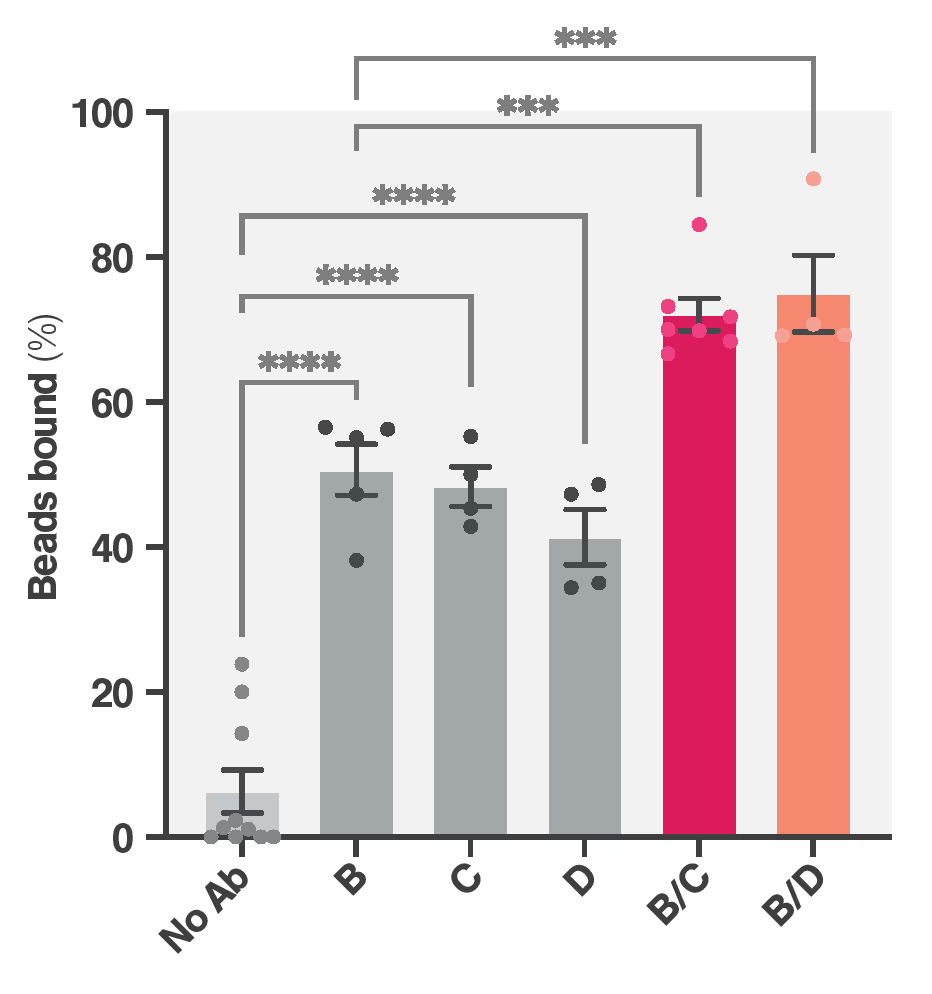
FGFR2 fusion genes confer sensitivity to FGFR2 kinase inhibitors like Pemigatinib, which saw accelerated FDA approval. However, response rates are hampered by tumor resistance from arising FGRF2 mutations. The researchers showed that the ECD of FGFR2 is necessary for a full oncogenic transformation by FGFR2 fusion. This makes the ECD a promising target for antibody therapies. Biparatopic antibodies present a promising opportunity for targeting FGFR2 owing to increased binding, specificity, and lower likelihood of inter-protein crosslinking. It was hypothesized that the most effective biparatopic antibodies would show both high affinity and high Cell Avidity.

Overview of the experimental setup. Left: Visualization of the monospecific antibody epitope sites (A-F) mapped to the FGFR2 extracellular domain (ECD). Center: workflow representation of the generation of the 15 biparatopic antibodies (A/B to E/F) from their monospecific constituent parts (illustrated by F40SL + K409R) by controlled Fab-arm exchange. Right: Viability assay output comparing the 15 biparatopic antibodies with the 6 monospecific antibodies (and an IgG1 negative control) on their growth inhibition of FGFR2-fusion-driven BaF3 cells.
Dive into the publication
Identification of potent biparatopic antibodies targeting FGFR2 fusion driven cholangiocarcinoma
Avidion
The next generation Cell Avidity platform


Avidigo
White glove Cell Avidity services

z-Movi
For small sized Cell Avidity studies
These cards are NOT components because they use the finsweet nested collection logic. To pull in the posible multiple people wo worked on it.
This type of 1-many relation is not supported native in Webflow.
Also this section is hidden when emtpy. To keep everything visible here, that is being done outside the webflow designer from within Slater.
Identification of potent biparatopic antibodies targeting FGFR2 fusion driven cholangiocarcinoma
SITC 2025
CAR-TCR Summit 2025
CICON 2025