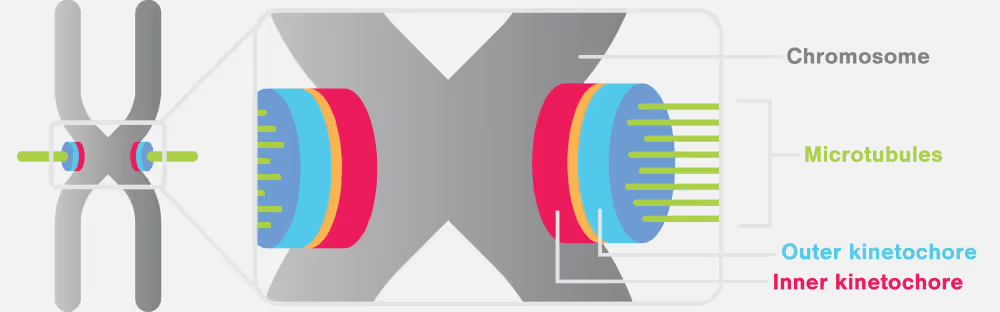
Errors in chromosome segregation can lead to profound genetic abnormalities, paving the way for various genetic diseases and developmental disorders. The kinetochore is one of the most sophisticated protein complexes in the cell and is fundamental to the accurate division of genetic material, sitting at the heart of the cell division process.
Despite decades of extensive research, the mechanosensitive properties of kinetochore’s function, and how it acts as a failsafe mechanism to prevent incorrect chromosome segregation, have remained elusive. The complexity of dynamic molecular interactions, particularly under the influence of mechanical stress during cell division, has posed challenges to scientists.

A recent landmark study published in Science journal led by Dr. David Barford from the MRC-LMB in Cambridge has utilized state-of-the-art LUMICKS C-Trap technology together with Cryo-EM to uncover new insights into this complex mechanism which is essential for life.
First, using Cryo-EM and AlphaFold2, key structural insights of the kinetochore microtubule attachment were provided. A set of key interacting domains were identified with a potential critical role to confer the ability of stable attachment between the kinetochore and the microtubule.
However, the extent to which these interfaces directly contribute to the force-resistance mechanisms remained an unanswered question. To address this, the authors utilized the C-Trap technique to directly demonstrate how the mutations influence the kinetochore’s ability to withstand microtubule forces during cell division.
Key proteins involved in the kinetochore and chromosome segregation
- Ndc80 complex: pivotal in cell division, accurately segregates chromosomes. It structurally assembles the kinetochore, congresses chromosomes, and signals spindle checkpoints. Directly binding microtubules, Ndc80 attaches them to chromosomes, playing an essential role in the dynamic attachment and movement of chromosomes, crucial for their correct alignment and distribution during cell division.
- CENP-A: A histone H3 variant, identifies centromere locations, guiding kinetochore assembly. Unlike typical nucleosomes, it loads independently of DNA replication, often in repetitive DNA regions, and is epigenetically replicated, ensuring consistent positioning in daughter cells without relying on the specific DNA sequence.
- DAM1 complex: A 210-kDa heterodecamer, plays a key role in chromosome motion during cell division. It self-assembles into microtubule-encircling rings, gliding along dynamic microtubule ends. Its interaction with the elongated Ndc80 complex tethers it to the kinetochore.
- Aurora B kinase: a serine/threonine kinase critical for mitosis, ensures accurate chromosome segregation and biorientation on the spindle. Its overexpression in cancers correlates with poor prognosis, making it a target for therapeutic inhibitors. It is vital in error correction during anaphase, interacting with tension-sensing mechanisms for chromosome attachment regulation.
By reconstituting the kinetochore protein complex from budding yeast in vitro, the MRC-LMB team successfully mimicked the natural conditions of chromosome segregation under mechanical stress. This experiment has demonstrated the critical role of the kinetochore-microtubule interface in kinetochore attachments, unveiling novel mechanisms that ensure kinetochore-microtubule stabilization and accurate chromosome segregation.

Key research highlights
The MRC-LMB work by David Barford’s team provides a quantitative assessment of the outer kinetochore’s force resistance of the Dam1 and Ndc80 complexes.
They have shown that this interaction is vital for the kinetochore’s ability to withstand forces encountered during microtubule dynamics in cell division. The rupture forces determined indicate that the kinetochore Dam1c:Ndc80c complex can endure significantly higher forces than Ndc80c alone, reinforcing the importance of their interaction for chromosome stability.
The mutations at the interface between Dam1C-ter and Ndc80cCH, and those affecting Dam1c ring assembly, were found to substantially reduce rupture forces, which highlights the individual contributions of these interfaces to microtubule binding strength. These findings align with the cell’s Error Correction (EC) mechanisms, which act by disassembling these outer kinetochore elements to prevent incorrect chromosome segregation.
Combining Cryo-EM and C-Trap techniques, this research connects the structure of kinetochore and the molecular mechanics behind kinetochore-microtubule attachments and provides vital insights into the error correction process in genomic stability and cell division.
K. Muir et. al., 2023, Science, 382, 1184-1190, December 12, 2023
“Structural mechanism of outer kinetochore Dam1-Ndc80 complex assembly on microtubules”