Tau is an intrinsically disordered microtubule-associated protein (MAP) that helps stabilize microtubules by forming an envelope around them. This stabilizing protein has been implicated in neurodegenerative diseases such as Alzheimer’s, where abnormal chemical changes promote detachment of tau from microtubules and the formation of long threads that eventually lead to tangles inside neurons. The mechanism of tau envelope formation has remained largely unknown up until recently. Now, in a Nature Chemical Biology study led by Zdenek Lansky from the Institute of Biotechnology of the Czech Academy of Sciences, researchers have shed light on the dynamics that govern tau envelope assembly on microtubules. Performing dynamic single-molecule experiments using LUMICKS’ C-Trap enabled the scientists to describe, in detail, how the formation and disassembly of the tau envelope depend on the characteristics of the microtubule lattice. Specifically, the lattice spacing between tubulin dimers dictates whether the envelope assembles or not.
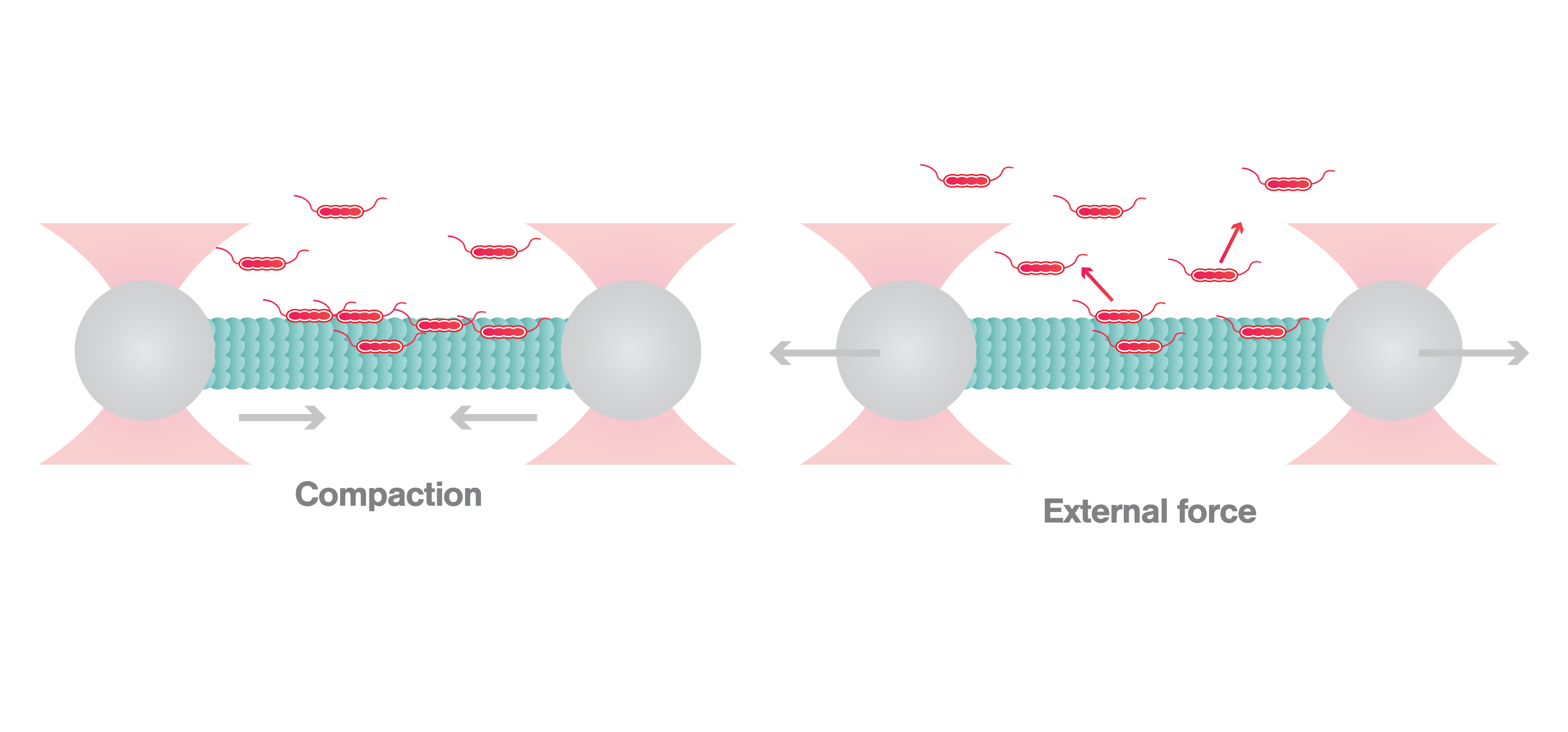
Initially, Siahaan and colleagues demonstrated that a compacted lattice of GDP-linked tubulin, representing the stage when the microtubule is not extending, induces envelope formation. In contrast, extension of the microtubule causes envelope disruption. To further investigate envelope formation at a molecular resolution and in real time, the researchers then used the C-Trap to examine whether global extension of the microtubule led to envelope disassembly. To this end, the two ends of a microtubule were attached to beads, both held by laser traps. The optical traps were then moved away from each other to create tension but without stretching the microtubule. When tau was added, the researchers measured an increasing force pushing the beads towards each other, representing the shrinkage of the microtubule due to the formation of the envelope. Then, they examined a microtubule with the tau envelope already formed and found that when the microtubule was stretched by pulling the two beads apart, the tau envelope disassembled twice as fast compared to the control experiment, i.e., when the microtubule was not stretched.
Based on the results of the C-Trap experiments, Siahaan and her colleagues concluded that the tubulin lattice state determines the formation and disassembly of the tau envelope. This suggests that tau is mechanosensitive — it senses the state of the lattice and behaves accordingly.
This study is a fine example of how dynamic single-molecule experiments with the C-Trap can be used to study the mechanical properties of microtubules. These findings could have important implications in the understanding and treatment of neurological disorders by preventing tau envelope detachment and microtubule destabilization.
For further information about this study, check out the research article “Microtubule lattice spacing governs cohesive envelope formation of tau family proteins,” published in Nature Chemical Biology.
Are you interested in using dynamic single-molecule tools like the C-Trap for your research? Do not hesitate to contact us for more information, a demo, or a quote.
Top image adapted from Siahaan et al., Nature Chemical Biology, 2022.