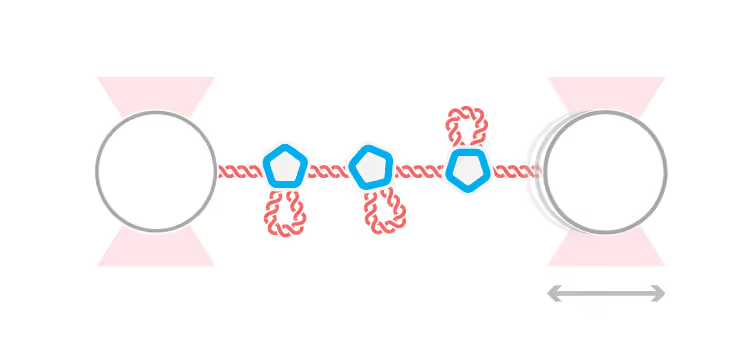
The research lab of Dr. Gijs Wuite in the Netherlands recently published a single-molecule study in Science Advances, demonstrating the progressive self-assembly steps of viruses. Methods, such as optical tweezers and acoustic force spectroscopy (AFS®), enabled the researchers to study the transient and otherwise hard-to-verify steps associated with viral assembly.
Understanding the detailed mechanisms underlying viral assembly can aid researchers in improving the encapsulation and delivery of various therapeutic agents.
Although previous studies have investigated capsid formation and viral assembly, they have not been able to record the transient procedure of virus-like particle (VLP) formation. Instead, our understanding of the procedure is based on models developed from end products of such procedures.
In the present single-molecule study, Rosmalen et al. could record the assembly in real time from start (capsid proteins binding naked DNA) to end (VLP formation). The team exposed single DNA-molecules to VP1, the major capsid protein of SV40 model virus, to evaluate its binding and assembly into virus-like particles. For example, the sensitivity of the high-throughput single-molecule AFS instrument enabled them to measure the minimal forces responsible for inhibiting DNA compaction.
Measured changes in DNA length and DNA–protein kinetics upon VP1 binding revealed that virus-like particle formation progressively increases the stability of protein interactions to create energetically favorable arrangements. In short, individual VP1 proteins compact the DNA through loop formation to subsequently accumulate and rearrange themselves on the molecule.
“The multistep assembly mechanism we revealed here provides insight in different molecular dynamics that lead to the formation of […] infectious particles and thus might guide the optimization of the production of SV40-based VLPs for therapeutic applications,” the authors concluded.
Congratulations to Dr. Gijs Wuite at the Vrije Universiteit Amsterdam, his lab, and all the authors involved in this work!
For more information, read the full article published in Science Advances titled “Revealing in real-time a multistep assembly mechanism for SV40 virus-like particles”.
Are you interested in using dynamic single-molecule tools like the C-Trap® or the AFS® for your research? Please feel free to contact us for more information, a demo, or a quote.