C-Trap Accelerator Suite
Data collection
Multiplexed experiments and enhanced workflows deliver unprecedented throughput
Faster set up
Guided wizard eliminates human error and improves reproducibility
More experimental time
Enhanced workflows dramatically increase productivity

Seven breakthrough upgrades for your LUMICKS C-Trap
C-Trap Accelerator Suite systematically targets every research bottleneck across your entire workflow: from sample preparation and instrument setup through workflow automation, experimental multiplexing, data analysis, training, and quality control. This complete ecosystem transforms both data quantity and quality, revolutionizing single-molecule research productivity.
“It’s a game changer, we couldn’t live without!”

Double Microfluidics Module
Maximize instrument utilization
Two independent microfluidics modules maximize data acquisition time by enabling flowcell cleaning in parallel with experimental execution, unlocking true back-to-back experiments. Parallel cleaning and measurement dramatically increases C-Trap throughput without compromising reproducibility.
DNA Repeat Assembly Kit
Up to 50x data throughput
A breakthrough DNA assembly kit multiplies experimental throughput by incorporating up to 50 identical binding sites into a single DNA construct. Collect up to 50x more binding events per experiment, shortening weeks of measurements into a single day data collection.


DNA Golden Gate Assembly Application Note
Combine multiple DNA sequences in a single experiment
Golden Gate technology allows you to seamlessly ligate multiple DNA fragments in any desired order into a single backbone and test multiple target DNA sequences within one single C-Trap experiment. This enables assays like DNA sequence-specific looping and significantly accelerates sequence screening workflows.
C-Trap Reference Kit
Your new standard assay for training
Fast-track C-Trap user training and proficiency, support assay troubleshooting, and verify instrument performance with the C-Trap Reference Kit. The kit provides standardized and validated reagents for a complete DNA-protein binding assay, including data analysis with reference values.


New protocol for Beads and Cleaning Kit
Improved experimental success & data quality
Our new streamlined cleaning and passivation protocol with optimized reagents secures pristine channels and consistent single-molecule data. The 60-minute protocol removes all adsorbed proteins and chemicals and re-passivates the flow cell, extending its lifetime and improving reproducibility.
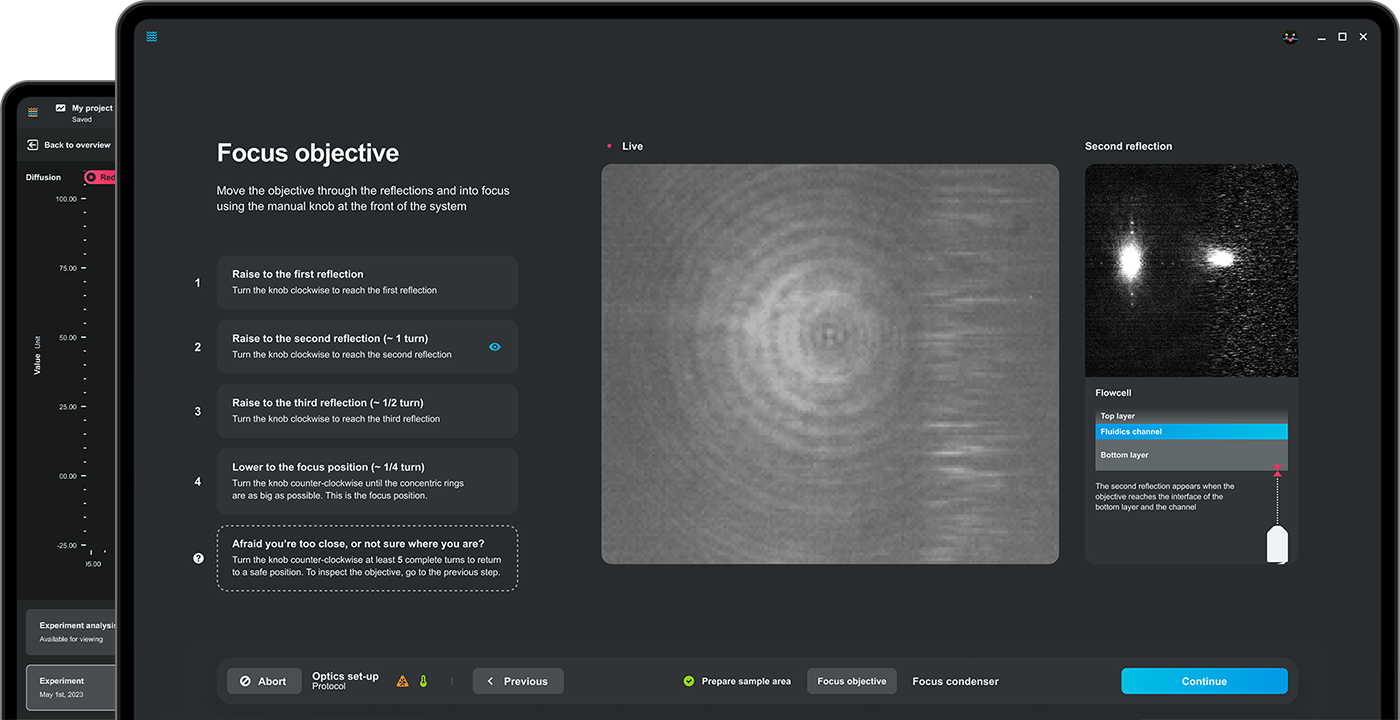
Bluelake 2.7: Guided Setup
50% faster instrument setup time
The on-screen guided setup guarantees optimal setup, boosting confidence for new and returning users. The visual wizard walks users step-by-step through the setup process, all the way up to the start of the experimental session, cutting setup time in half with zero guesswork.
Update available as part of your service contract
Don't have a service contract?

Lakeview 1.4
Transform 6 hours of data processing into just minutes
Automated analytics convert raw C-Trap data into clear, publication-ready insights within minutes. The pre-built analysis pipelines extract key events and enable export figure-ready data and movies in one click.
Update directly through Lakeview
Don't have Lakeview yet?
Watch the webinar

Single-molecule data at warp speed: 3,905 events in 2 hours
Here's how the full C-Trap accelerated workflow pays off. Using DNA repeat assembly kit, Bluelake, and Lakeview data analysis, LUMICKS researchers captured thousands of high-quality kinetic datapoints in a single afternoon session.
The Experiment
- Sample: DNA repeat assembly generated 100kb-long DNA including 50 identical LacO operator sites
- Automation: Bluelake automatically caught beads, formed tethers, and recorded kymographs non-stop for hours
- Data: 3905 individual LacI binding events were measured in 2 hours of experimental time
- Analysis: Lakeview analysed tens of Gb of data in less than 2 hours
- Insight: DNA Bound lifetime was accurately determined to 2.75s +- 0.3s
Key insights
- One afternoon, one operator: Minimal hands-on time required
- Thousands of datapoints: Statistical power previously impossible
- Fully integrated workflow: Sample preparation, acquisition, and analysis seamlessly connected
- Publication-ready results: High confidence from robust statistics

DNA Repeat Assembly Kit
- 100kb DNA Repeat
- 50x LacO Sequence Repeat

Bluelake & Lakeview
- Bluelake Data Acquisition enables 2hrs of complete experiment automation
- Lakeview Data Analysis analyzes lifetime of 2.75s +/- 0.3s, N = 3905
What's next?
LUMICKS University
Our new gateway into the single-molecule ecosystem
Our new interactive learning hub equips every user with step-by-step C-Trap expertise on demand. Bite-size modules and interactive materials turn novices into confident and proficient C-Trap single-molecule operators.
- 📚 Written articles
- 🎥 Video tutorials
- 📰 Product news
- 📖 Support documentation
- 📋 Product manuals
- 💾 Software releases
Coming next quarter!

Filter DSM and show 4 latest
This shows the most recent card of each resource type filtered on Business Unit
Webinar, Scientific update, Whitepaper, Application note, Brochure.
We only show 4 and we have 6 types so the 2 older ones are hidden.
In design only 1 is shown, but the rest will be loaded when published.
Single Molecule Visualisation of Human Topoisomerase 2A Decatenation Reveals Substrate Requirements
DNA replication introduces double-stranded DNA entanglements, which pose a challenge to cell division and faithful segregation of the genome. During mitosis, Topoisomerase 2A (TOP2A), binds and resolves DNA entanglements by producing a double strand break and then passing the other strand through the break. TOP2A is an essential protein and an important drug target, hence has been extensively studied, but until now the resolution process has not been directly visualised. In this work, I develop an assay for visualising TOP2A activity, mimicking forces that could be applied in a mitotic context, by employing optical tweezers to manually entangle two pieces of DNA(1). I demonstrate that TOP2A DNA resolution is inhibited at high forces, with sharp transition at the half-force of 28 pN. My experiments indicate TOP2A readily binds DNA, however I find resolution is most efficient when TOP2A associates directly at the site of a DNA entanglement. During early mitosis the action of TOP2A chromosome resolution is countered by cohesin and I demonstrate that cohesin readily associated with entangled DNA, and inhibits TOP2A resolution, implicating TOP2A in regulating chromatid cohesion. Collectively, this approach provides novel insights into the important therapeutic target, TOP2A(2).
(1) Meijering, A.E.C., Bakx, J.A.M., Man, T., Heller, I., Wuite, G.J.L., and Peterman, E.J.G. (2022). Implementation of 3D Multi-Color Fluorescence Microscopy in a Quadruple Trap Optical Tweezers System. In Methods in Molecular Biology, pp. 75–100. 10.1007/978-1-0716-2229-2_5.
(2) Cutts, E.E., Saravanan, S., Girvan, P., Ambrose, B., Fisher, G.L.M., Rueda, D.S. and Aragon, L. (2025). Substrate accessibility regulation of human TopIIa decatenation by cohesin. Nature Communications, https://doi.org/10.1038/s41467-025-62505-3
Linking Mechanical Stability with in vivo Recombination: Single-molecule Research Reveals Bacterial Antibiotic Resistance
Golden Gate meets C-Trap: A powerful combination for unprecedented molecular insights
Precisely manipulating genetic material at the single molecule level is gaining importance across life sciences – and so do the tools that allow researchers to do exactly that. The C-Trap system combines single molecule fluorescence microscopy with optical tweezers to manipulate DNA, allowing researchers to directly observe and track molecular events as they occur. Designing and creating specific DNA constructs is crucial for maximizing the potential of single molecule studies. In this application note we introduce the powerful combination of cutting edge biochemistry and single-molecule visualization methods to increase throughput and maximize the results gained from each individual measurement.
C-Trap Product Brochure








